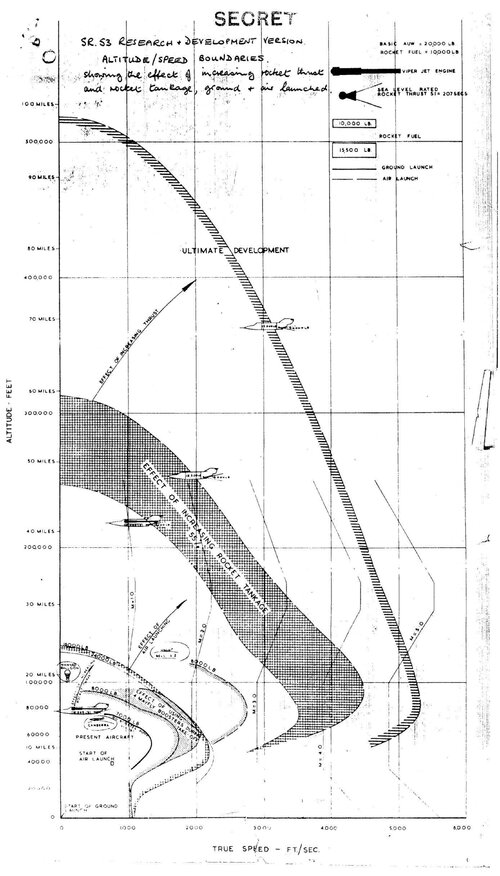
I've red a lot about that H2O2 stuff - and the record is definitively mixed and controversial.
Some points
- H2O2 needs to be 98% pure to get max specific impulse out of it (324 seconds is feasible)
- 85% or 90% instantly degrades the specific impulse to 280-290 seconds - although it improves handling and safety
- the purer the H2O2, the worst it reacts to impurities, including water vapor from the atmosphere
- The NF-104A (three built and flown for 8 years 1963-71) is typical of H2O2 mixed record
- It did not killed any pilot yet at some point in the late 1960's was a hangar queen because that H2O2 in the rocket created many structural issues
- H2O2 from 1945 to 1980 had some purity issues that led to severe accidents
- Yet from the moment it became part of hybrid rockets (besides solid fuel: AMROC, 1985) and then of Andrew Beal BA-2 project (1997), new manufacturing processes with more purity vastly improved H2O2 handling and safety
I have a whole bunch of documents on my HD if anybody is interested.
H2O2 was once my favorite "begnin" oxider for suborbital refueling rocketplanes; but since then I've learned about NYTROX which is a blend of N2O and LOX, lowering their freezings points (-183°C for LOX, -88°C for N2O). Nytrox could get a gentler -50°C without losing too much specific impulse.
But fact is that finding a non-deep-cryogenic, non-toxic, non-unstable oxidizer for rocketplanes is next to impossible. N2O sucks, H2O2 explodes, LOX is too cold, N2O4 is a corrosive and toxic b*tch of a substance. And with the aforementioned four, 90% of potential rocket oxidizers have been mentionned.
That list perfectly summarize the issue.
 en.wikipedia.org
en.wikipedia.org
What is not storable / hypergolic (N2O4 and others) is horrible and murderous stuff (fluorine & "a good pair of running shoes" in the words of John Ignition Clark) .
And once all the nasty substances are removed, all that's left are the trio LOX (deep cryogen) H2O2 (unstable explosive bastard) and N2O (suck at performance, mild-cryogen, and killed three Virgin / Rutan / Scaled employees in July 2007).
That's why nytrox is interesting: it very much tries to blend LOX & N2O into a more begnin substance to compete with H2O2 as "alternative oxidizer to sacrosanct LOX".
Some points
- H2O2 needs to be 98% pure to get max specific impulse out of it (324 seconds is feasible)
- 85% or 90% instantly degrades the specific impulse to 280-290 seconds - although it improves handling and safety
- the purer the H2O2, the worst it reacts to impurities, including water vapor from the atmosphere
- The NF-104A (three built and flown for 8 years 1963-71) is typical of H2O2 mixed record
- It did not killed any pilot yet at some point in the late 1960's was a hangar queen because that H2O2 in the rocket created many structural issues
- H2O2 from 1945 to 1980 had some purity issues that led to severe accidents
- Yet from the moment it became part of hybrid rockets (besides solid fuel: AMROC, 1985) and then of Andrew Beal BA-2 project (1997), new manufacturing processes with more purity vastly improved H2O2 handling and safety
I have a whole bunch of documents on my HD if anybody is interested.
H2O2 was once my favorite "begnin" oxider for suborbital refueling rocketplanes; but since then I've learned about NYTROX which is a blend of N2O and LOX, lowering their freezings points (-183°C for LOX, -88°C for N2O). Nytrox could get a gentler -50°C without losing too much specific impulse.
But fact is that finding a non-deep-cryogenic, non-toxic, non-unstable oxidizer for rocketplanes is next to impossible. N2O sucks, H2O2 explodes, LOX is too cold, N2O4 is a corrosive and toxic b*tch of a substance. And with the aforementioned four, 90% of potential rocket oxidizers have been mentionned.
That list perfectly summarize the issue.
Category:Rocket oxidizers - Wikipedia
Ammonium dinitramide
Ammonium nitrate
Ammonium perchlorate
Chlorine pentafluoride
Chlorine trifluoride
Dinitrogen tetroxide (N2O4: storable hypergolic Ariane / Proton / Titan / Long March oxidizer)
Hydrogen peroxide (H2O2)
Liquid oxygen (LOX)
Mixed oxides of nitrogen
Nitronium perchlorate
Nitrous oxide (N2O)
Oxygen difluoride
Perchloryl fluoride
Red fuming nitric acid
T-Stoff
Tetrafluorohydrazine
Tetranitromethane
Trinitramide
What is not storable / hypergolic (N2O4 and others) is horrible and murderous stuff (fluorine & "a good pair of running shoes" in the words of John Ignition Clark) .
And once all the nasty substances are removed, all that's left are the trio LOX (deep cryogen) H2O2 (unstable explosive bastard) and N2O (suck at performance, mild-cryogen, and killed three Virgin / Rutan / Scaled employees in July 2007).
That's why nytrox is interesting: it very much tries to blend LOX & N2O into a more begnin substance to compete with H2O2 as "alternative oxidizer to sacrosanct LOX".
Last edited: