Ammonia is pretty toxic in its own right, while gasoline is only moderately so. Although it is less flammable than gasoline, it is far more caustic; you really do not want it in your lungs for example. Because of this, localised ammonia storage has a lot more safety red tape than gasoline, you can't just fill up a plastic can and stick it in the back of your car. A cloud of gasoline fumes rolling over your town will cause more harm from the additives than from the gasoline fumes, but the same cannot be said for ammonia. The stuff was even used as a poison gas during WWI, though it was admittedly not very effective in the field.
Ah No ;-
- unleaded gasoline vapour is highly Carcinogenic due to its benzene content
https://www.sciencedirect.com/science/article/abs/pii/S0013935105802439
- Gasoline is heavier than air so it will roll along the ground. NH3 under most environmental conditions is lighter, so it will go up at point of release.
- I can find no reference to ammonia ever be used as a WW1 chemical warfare agent, please supply a source & reference. Under most circumstances it’s lighter than air, so it’s a bit like trying to gas someone in a trench with hydrogen/helium;- makes no sense to even try.
Despite your surprised opinion on NOX, it’s correct that NH3 can both be used to suppress NOX in conventional gas turbine combustion and if burnt directly produce lower NOX than fossil fuels. I can provide sources.
NH3 is the second most produced and transported chemical in the world today (approx 200 million tons a year) from brown carbon sources. However there are now a number of multi million ton/year fully funded, billion dollar schemes to produce Ammonia from sunlight/air/water;- the latest being In Saudi. It dissolves in water, where it naturally decomposes, so no more oil slicks in the event of a tanker leaks/sinkings.
The principal advantage is storage costs and it’s well to tank energy conversion;- there’s more hydrogen in a cubic meter of anhydrous ammonia than there is in liquid hydrogen.... yes really, google is your friend. NH3 storage cost about 5% compared to liquid hydrogen, has close to zero storage loss and requires 10-20% less energy to produce compared to energy available at point of use.
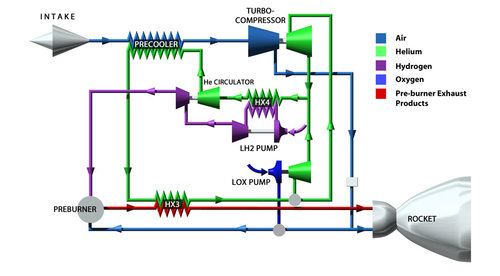
There’s no missing heat exchanger in the diagram, after it’s converted to gas it just burnt, as was proven by Prof Kobayashi gas turbine demonstrator,;-
https://www.ammoniaenergy.org/articles/ammonia-fueled-gas-turbine-power-generation/
I really can’t work out your thermodynamic logic at all.
The “least vile option rocket fuel”;- Rocketdyne Project Leader for the X15 XLR99 - Robert W Seaman actually said “I have worked with a quite a few propellant systems and found NH3/LOX to be among the easier ones to work with. We did encounter a few challenges along the way, but none were related to the fuel itself” ;-
I believe his assessment far more than yours.