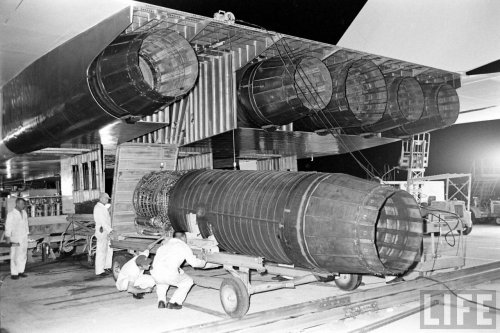
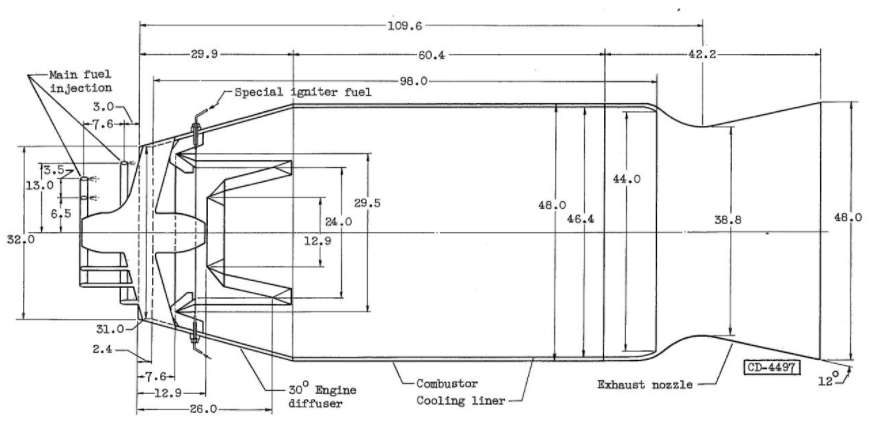
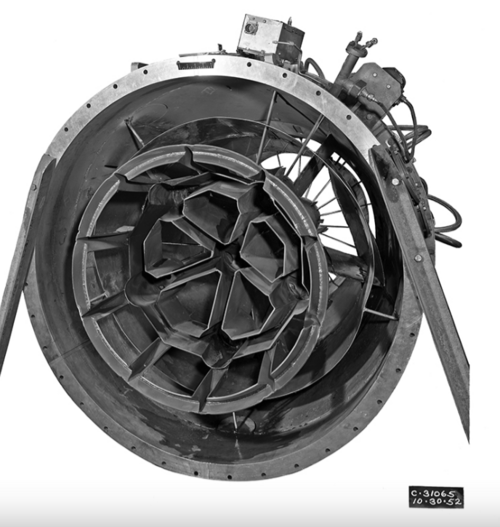
Fuel heat of combustion. - A convenient means of presenting the
fuel picture in relation to heat of combustion is to plot the heat of
combustion of the elements as a function of their atomic numbers (fig.
6). Heats of combustion in excess of the current value of JP-4, 2400
nautical-mile pounds per pound (approximately 18,500 Btu/lb), can be obtained
by substituting lithium, beryllium, or boron for the carbon of
hydrocarbons, or by eliminating these elements entirely and using
hydrogen.
Lithium is not enough better than carbon to be of much interest.
Beryllium is much rarer, and is more toxic than boron, which leaves the
boron-hydrides of major interest. Pentaborane (B5H9) has a heat of combustion
of 29,000 Btu per pound. The development of the boron fuels
under the code name of Zip is being actively sponsored by the Department
of Defense. A fuel consisting of a combination of boron-hydride and
hydrocarbon with an estimated heat of combustion of 25,000 to 26,000 Btu
is being produced in laboratory quantities. If this fuel can be used in
place of JP-4, a range increase of 40 percent (26,000/18,500 = 1.40)
will be realized. However, the combustion products, boron oxide, tend
to deposit as a solid in the combustor and on the turbine stator blades;
intensive research is needed on this problem. Without going into details,
current research indicates that combustion of Zip fuel in the
afterburner causes less trouble than combustion in the primary combustor.
Use of Zip fuel in the afterburner only will increase range about 25 percent,
for that portion of the flight in which the afterburner is used.
Research and development on Zip fuel is currently limited in scope
because of the small quantities of the fuel that have been available.
Based on present recommedndations of the Department of the Navy and Department
of the Air Force, sufficient fuel should be available in about
2 years to permit an adequate attack on the problem of exhaust product
deposits. In the mean time, interesting laboratory results on full scale
engines are being obtained with the limited fuel quantities now available.
Hydrogen as a fuel would give a heat of combustion 2.75 times that
of JP-4, and would present no major engine operation problems,, In fact,
because of its combustibility, hydrogen has good combustion efficiency
at altitudes much in excess of those currently being used. The principal
disadvantage of hydrogen is its low density; in liquid form hydrogen
is only one-tenth as dense as JP-4, and extremely low temperatures are
required to maintain the liquid form. In comparison with a quantity
of JP-4 of given energy content, an equivalent amount of hydrogen would
weigh 0.4 as much, but would occupy four times the volume. This
lower density, with consequent larger fuel tanks, has led to consideration
of hydrogen primarily for altitudes above 70,000 feet and generaly
for radii of action less than that required for the strategic bomber
mission. Current interest in this fuel is for flights at considerably
higher altitudes, with particular emphasis on the reconnaissance
mission. The Department of the Air Force, in conjunction with the NYACA,
is conducttig an accelerated program on the use of hydrogen. Use of
hydrogen is discussed more fully in references 2 and 3.
Summarizing the chemical fuel picture: zip fuels may well increase
potential range of the strategic bomber by 25 percent of the portion of
the flight in which an afterburner is used. A potential range increase
of 40 percent will be realized if the boron oxide deposit problem is
solved, permitting full use of Zip fuel. The low density of hydrogen
makes it of current interest as a fuel to be used at quite high alti-,
tudes; decision on its use as a long-range fuel must await additional
research.
If the strategic bomber is powered with nuclear fuel instead of
chemical fuel, the value of h becomes many orders of magnitude greater
than that for chemical fuels. In this case, because range is sufficiently
greater than that required by the bomber mission, other factors,
notably nuclear radiation effects on the crew, determine the time the
airplane can stay in the air and so determine the radius.